Revolutionizing the root canal

Dental pulp regeneration is the end goal of this $1.8 million NIH grant awarded to TAMBCD researchers
More than 15 million root canals are performed in the U.S. every year. The underlying reasons are many and varied: deep decay, trauma, fractures, faulty crowns and the cumulative effect of repeated dental procedures. The end result — infected and inflamed tooth pulp — is what a root canal aims to treat.
During this treatment, endodontists remove damaged pulp, disinfect the inside of the tooth, and fill and seal it with gutta-percha, a rubber-like material. Finally, the tooth is restored and protected with a crown or filling.
A synthetic biomaterial developed by Texas A&M University Baylor College of Dentistry researchers could be utilized in much the same way as gutta-percha, but with very different results. With a $1.8 million, five-year National Institutes of Health-National Institute of Dental and Craniofacial Research grant set to begin in July, Dr. Xiaohua Liu will further develop a tissue engineering strategy that stands to revolutionize the modern-day root canal. The associate professor in biomedical sciences has fabricated a combination of dental pulp stem cells and tubular-shaped scaffolding materials that allow dental pulp to regenerate itself — correctly structured tissues, blood vessels and all. The outcome: a healthy, living tooth.

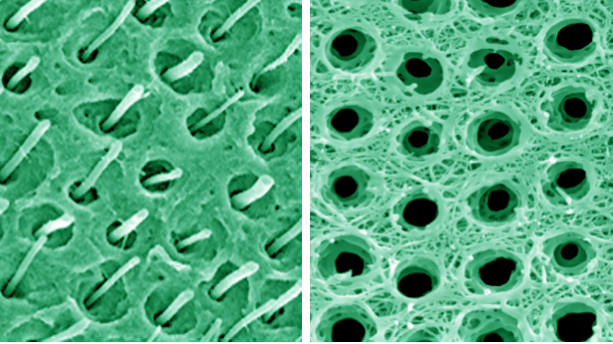
It’s a developing therapy that could stand to revolutionize root canal treatment as we know it, potentially impacting endodontists like Dr. Jianing He, adjunct associate professor and a consultant on the project.
“The current material used in root canal treatment does not strengthen the root nor does it provide any defense against bacteria invasion; it simply acts as a space filler,” He says. “The approach proposed in Dr. Liu’s grant will allow the patient to regenerate a pulp-dentin complex that is both anatomically and functionally similar to the original structure. This structure will allow continued development of the tooth, and just like the natural tissue, it will have its own defense mechanisms to protect the tooth against future infection.”
It’s a project several years in the making already. Liu explains one of the hurdles his team has had to address since starting the study in 2011.
“Our strategy is a lot like the root canal, but we actually try to regrow the living tissues inside. In that way, your tooth is still a living tooth. The challenge with regenerating dental tissue is that we don’t just want to have the cell; we have to make sure it is in the right structure,” Liu says. The reason: Without a well-organized structure, the regenerated tissues cannot perform normal mechanical and biological functions.
And the structure of dentin, that dense, bony tissue under enamel that houses the pulp, is very, very distinctive. It’s long and tuberous — picture your garden variety rhubarb, but 2 to 4 micrometers in diameter. The synthetic dentin matrix designed by Liu and his team is the first of its kind and forms the basis for vascularization tests, in which a human tooth root injected with the material is implanted under the skin of mouse models. The outcome could lay the groundwork for additional preclinical testing and, eventually, clinical trials.
Dr. Jerry Feng, professor and vice chair of biomedical sciences and a consultant on the project, shares another consideration when dealing with regeneration of dentin found in teeth as compared to regeneration of bone found elsewhere in the body.
“The major difference between bone and tooth dentin is that the dentin has an extremely low capability to repair itself when damage occurs,” says Feng. “In contrast, bone can completely repair itself in a few months without treatment, in most cases.
“Dr. Liu’s work, regeneration of dentin in vitro in a way similar to what’s happening in our body, is a very promising start for patients who have tooth fracture or diseases. This project is likely to change the future of dentistry if this man-made dentin is applied to human patients.”
Endodontists welcome the advancement’s groundbreaking potential.
“Regenerative endodontics is the frontier of our specialty,” says He. “The American Association of Endodontists recognizes its importance and has developed special committees and grants to support the research and development in this field; however, there has not been a lot of NIH funding for this type of research. Therefore it is especially exciting to see Dr. Liu’s grant get funded.
“Our goal is to develop a predictable treatment approach that can be used on a broad patient base, and to have endodontists perform these procedures in the future.”